In organizations that comply with Quality Management Systems (QMS) - whether under ISO 9001, ISO 13485, or other regulated frameworks - there’s an ongoing debate: When do you log an NC, when do you initiate a CC, and when do you escalate to a CAPA?
While NC (None-conformity), CC (Change Control), and CAPA (Corrective and Preventive Action) are core tools in QMS, their proper use is not always clear in daily operations. Misusing them can lead to wasted effort, delayed product releases, or missed systemic fixes. This post breaks down each concept, compares them, and explains where TDA (Temporary Deviation Approval) fits in.
Let’s Review the meaning of each one:
NC (Nonconformity)
A Nonconformity is any failure to meet a requirement - whether it’s a product specification, process standard, regulatory obligation, or customer expectation.
- Purpose: Identify and document that something went wrong.
- Example: Dimensions out of tolerance, missing inspection records, or an audit finding.
CC (Change Control / Change Request)
A Change Control (sometimes called a Change Request) is a formal process to propose, review, approve, and implement changes to controlled product, process, or documentation.
Purpose: Ensure changes are evaluated for their impact on quality, safety, compliance, and customer requirements before they’re implemented.
Typical Steps:
- Submit change request form.
- Impact assessment (quality, regulatory, cost, timing).
- Approval by authorized stakeholders.
- Implementation and verification.
Example: Updating a device drawing to change a material, modifying a production process to add an inspection step, or revising a QMS procedure.
CAPA (Corrective and Preventive Action)
A CAPA is a structured process to eliminate the root cause of an existing nonconformity (Corrective Action) and/or prevent a potential nonconformity (Preventive Action).
Purpose: Solve systemic issues and prevent recurrence/occurrence.
Example: Updating supplier requirements and training programs after repeated audit failures, adding a fail-safe feature to a part to prevent assembly errors, like a USB that can be inserted only in one way.
Use Case Example
A medical device company receives a supplier notification that a critical part will no longer be manufactured in its current form due to raw material changes.
NC: If parts are received and don’t meet the current specification, it’s logged as a nonconformity.
CC: A formal Change Control is opened to update the specification, drawings, and validation documents to accommodate the new material.
CAPA: If the change was driven by recurring failures in the old design or supplier quality issues, a CAPA may be triggered to address the root cause and prevent similar issues.
Differences Between NC, CC, and CAPA
NC, CC, and CAPA are all essential in QMS, but they serve different purposes. NC deals with what went wrong, CC manages planned changes, and CAPA addresses why something went wrong and how to prevent it. We’ve placed all these differences in a table below to bring clarity and order to the different processes names.
What ISO 9001:2015 and ISO 13485:2016 Say About NC, CC, and CAPA?
Where TDA (Temporary Deviation Approval) Fits In
TDA (often called a concession in ISO) is a formal, time-limited approval to accept a product or process that does not fully meet requirements, under controlled conditions.
Standards References:
ISO 9001:2015 - Clause 8.7: “…authorized for use, release or acceptance under concession…”
ISO 13485:2016 - Clause 8.3.3: “…may be accepted by concession provided it has been authorized…”
Example: Supplier notifies you before shipment that parts are slightly out of tolerance; you approve them for a specific batch to prevent a production delay.
Key point: TDA is not the same as CC or NC - it’s an approval decision linked to an NC when you choose to accept the deviation rather than correct it.
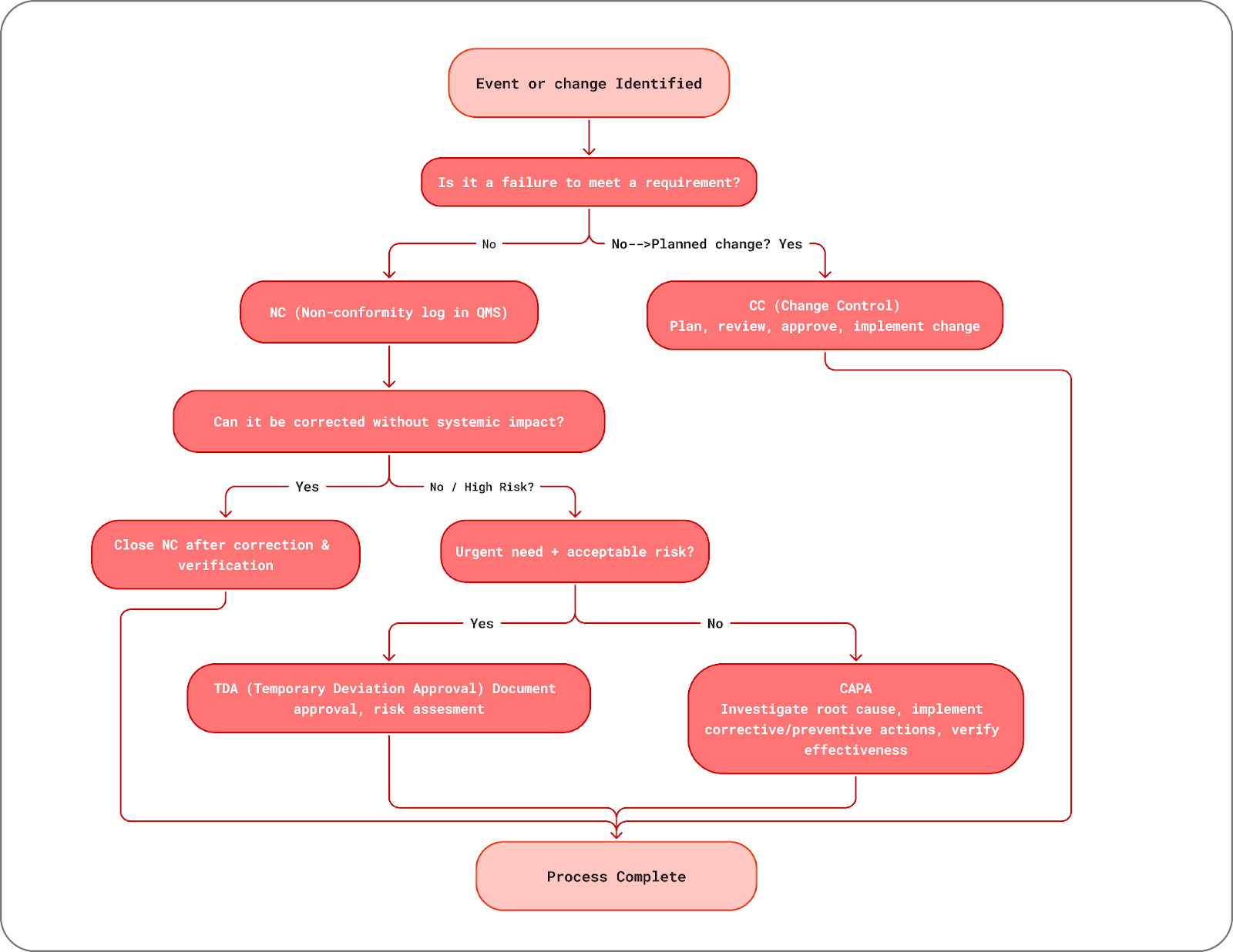
NC, CC, CAPA, TDA - Decision-Making Guide
To make it easier to determine the right path when an event, deviation, or change arises, we’ve created this decision flow diagram. It visually guides you through the process of deciding whether the situation should be managed as a Nonconformity (NC), handled through Change Control (CC), escalated to a Corrective and Preventive Action (CAPA), or approved under a Temporary Deviation Approval (TDA). By following this flow, you can ensure that every decision is consistent, compliant with ISO requirements, and documented appropriately.
Our Take
Managing changes and nonconformities - especially in medical devices and pharma - is complex. At bananaz, we make it easier. We help you track model and drawing changes, enforce design protocols, and use checklists to stop human errors before they happen. With our AI-powered tools and advanced exports, you can create clear, audit-ready change documentation in minutes, maintain a complete audit trail, and communicate changes seamlessly.


.png)
.png)

